This page brings together the main information on hydrazine (N2H4) or "diazane" such as its physical characteristics, its effects on health, the means of detecting it (N2H4 gas detector) as well as the appropriate respiratory protection equipment (gas mask or assisted ventilation device with combined K-P3 filter).
Best known for its use as a rocket fuel, hydrazine (N2H4), or " diazane ", is used in the chemical and pharmaceutical industries as a synthesis intermediate and in the rubber industry as a blowing agent in the manufacture of polymer foams. Its properties, particularly its liquid state at room temperature, are popular in the manufacture of fuel cells.
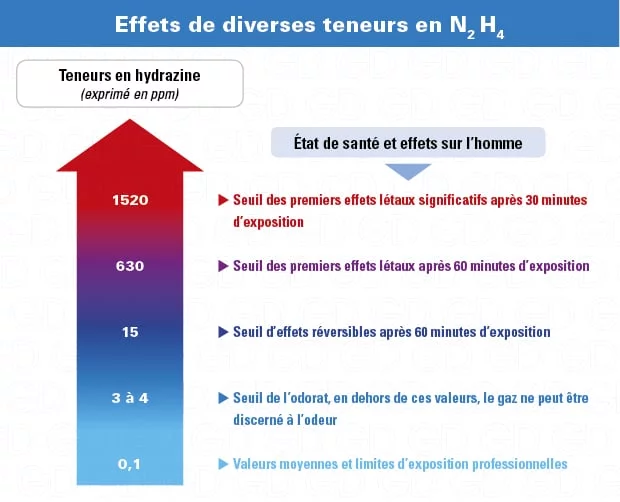
Colorless , with an odor reminiscent of ammonia at very low concentrations, hydrazine (N2H4) is very soluble in water or alcohols that can form explosive vapors. Although flammable in both liquid and gaseous states (H226), it is a very toxic gas by inhalation, ingestion or skin contact (H331/311/301) and classified 2B (potentially carcinogenic) by the IARC* . It can also cause skin burns and eye damage (H314).
*IARC: International Agency for Research on Cancer (part of the WHO World Health Organization)
Indistinguishable by smell, only a N2H4 gas detector can accurately measure the concentrations of this gas. Although highly explosive (1.8% volume), an explosiveness measurement is useless with this extremely toxic gas, so we will move towards a portable or fixed hydrazine detection .
Hydrazine is an eye irritant, so a full face mask is recommended for short-term interventions or a more comfortable powered ventilation device with combined K-P3 filters . If concentrations exceed 60 times the OEL, a self-contained breathing apparatus will be essential.
check_circle
check_circle
Nous utilisons des cookies tiers pour améliorer votre expérience de navigation, analyser le trafic du site et personnaliser le contenu et les publicités. En savoir plus