This page brings together the main information on freons, more commonly called refrigerant gases or refrigerant fluids, such as their physical characteristics, their effects on health, the means of detecting them (freon detector) as well as the appropriate respiratory protection equipment.
Refrigerant gases were born for the preservation of food. The first to be used were: carbon dioxide CO2, sulfur dioxide (SO2), chloroethane (C2H5Cl) , chloromethane (CH3Cl), ammonia (NH3) and some hydrocarbons.
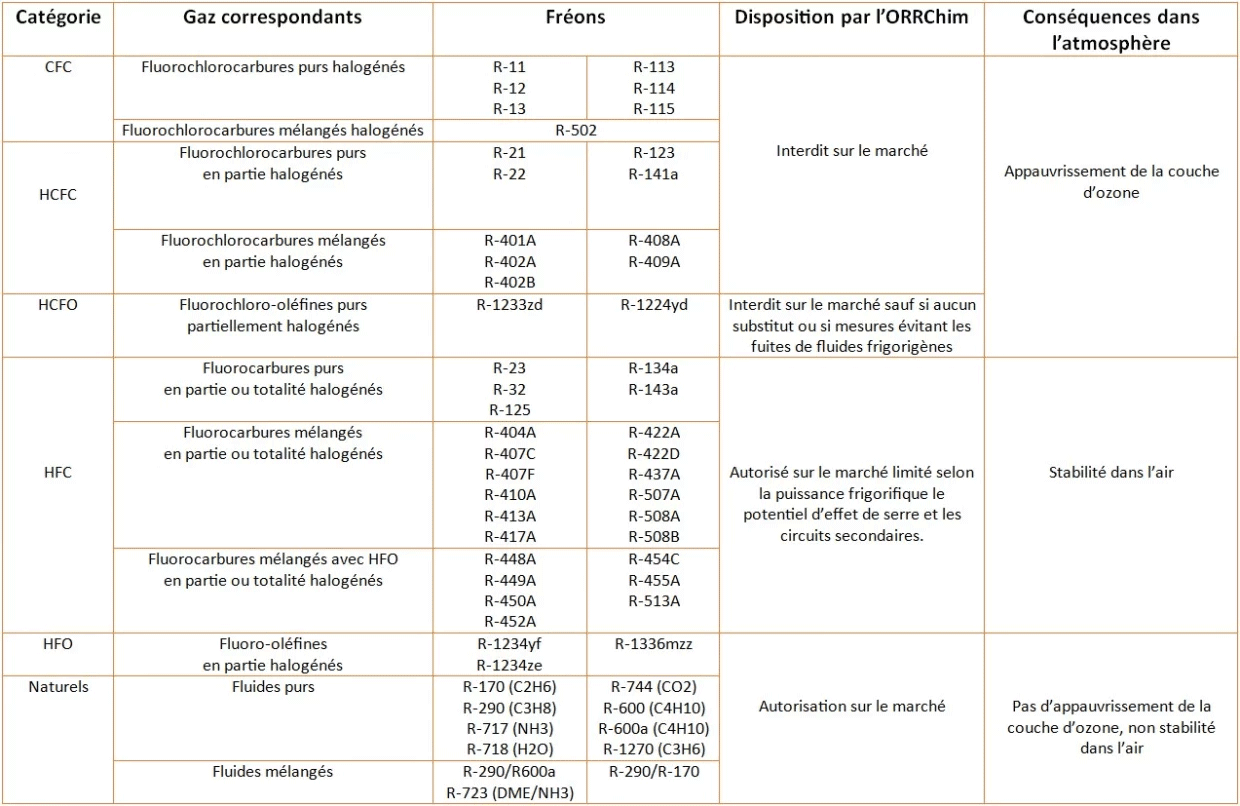
We then find many other refrigerants classified according to their properties:
CFCs: Chlorofluorocarbons are halogenated fluorinated gases composed of carbon (CO), chlorine (Cl) and fluoride (F) atoms.
HCFCs: Hydrochlorofluorocarbons are hydrogenated CFC gases that act as greenhouse gases in the depletion of the ozone layer. Therefore, their production and use are prohibited.
HFCs: Hydrofluorocarbons are third-generation refrigerants replacing freons banned on the market. More efficient, they are less harmful and have a better impact on the environment.
HFOs: Hydrofluoroolefins are the fourth generation of gases. They are very low pollutants and are therefore very suitable for air conditioning and commercial refrigeration.
Among the natural gases that have refrigerant properties, the most widespread is ammonia. It is the reference refrigerant gas in industrial refrigeration systems, in large pump systems, the food industry, and high-power industrial chillers.
Initiated in 1997, the Kyoto Protocol was ratified in 2005 by 55 countries committed to reducing greenhouse gas emissions: carbon dioxide – CO2, methane – CH4, nitrous oxide – N2O, hydrofluorocarbons – HFC, perfluorocarbons – PFC, sulfur hexafluoride – SF6.
Toxic, explosive or asphyxiating, refrigerant gases represent a potential danger for people and property in the event of a leak. This is why it is essential to use a refrigerant gas (freon) detection system to optimize the safety of people and avoid costs incurred by refrigerant leaks.
check_circle
check_circle
Nous utilisons des cookies tiers pour améliorer votre expérience de navigation, analyser le trafic du site et personnaliser le contenu et les publicités. En savoir plus